
KoSAR prospectively collects and monitors treatment response and disease progression of patients with severe asthma on regular follow ups.
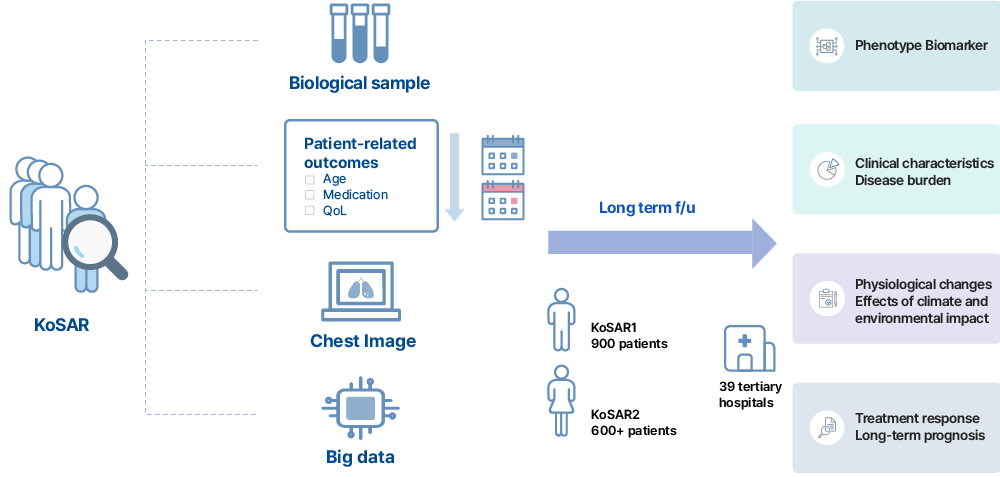
Demographic and clinical information including gender, age, living environment, substance use, comorbidities, pulmonary function test, and laboratory test results.
Captured data will be stored into the eCRF.
Plasma and DNA from the participants
in accordance with the Human Specimen Collection guideline and business manual of National Biobank of Korea (NBK).
High-resolution chest CT images
At the time of enrollment and regular followups
To evaluate the long-term prognosis and disease burden of patients with severe asthma
The data will be examined in connection with claims data and death data of the Health Insurance Review and Assessment Service (HIRA) and the National Health Insurance Service (NHIS) for research participants with informed consents.
KAAACI Working Group on Severe Asthma